Contact us right away
If you run into any issue or have any question.
 E-mail: michael@shboqu.com | Expert in Water Quality Measurement and Water Treatment Project
E-mail: michael@shboqu.com | Expert in Water Quality Measurement and Water Treatment Project
pH meters are a technology that, while rooted in designs from the 1930s, has significantly evolved. Today, we have access to various automated pH meters that streamline the process of pH measurement.
A pH meter is a tool that evaluates the acidity or basicity of a solution. Solutions with an acidic nature possess an abundance of positively charged hydrogen ions, whereas basic (alkaline) solutions are rich in negatively charged hydroxide ions.

The fundamental principle guiding ph water meters is known as potentiometry, which involves measuring a solution's electrical potential (or voltage). Consider acidic solutions – their high hydrogen ion content means they can conduct electricity well, a property known as electrical potential. This concept is crucial in both understanding and utilizing pH meters.
In practice, a ph water meters uses two electrodes inserted into the liquid, forming an electrical circuit. The reference electrode contains a substance with a known electrical potential, while the sensor electrode is immersed in the test solution. The pH meter then measures the difference in electrical potential between these two electrodes.
pH meters find their use in a diverse array of fields, from medical research to industrial applications. The principle of measuring a solution’s voltage is consistent across these uses, which invariably involve liquid samples. In the pharmaceutical industry, ph water meters are indispensable for researching drug metabolism, where acidity plays a significant role. Agricultural practices benefit from ph water meters in testing soil and water quality. They are also crucial in water purification plants for validating filtration techniques and ensuring water safety in swimming pools and aquariums. Beyond these, ph water meters are integral in developing food products, cosmetics, and detergents, showcasing their extensive applicability.
Some of the parts that make up a ph water meters are:
The High Input Impedance Meter
This component is essentially the brain of the device, housing a microprocessor adept at handling minuscule electrode voltages. It’s responsible for interpreting the pH levels of a solution, factoring in the measurement temperature, and converting the amplifier’s voltage readings into understandable pH units displayed on the screen.
The Combined Electrode
The combined electrode is central to the pH meter's functionality, comprising two critical parts where the actual measurement process occurs. This dual-electrode system, both intricate and delicate, is the most crucial yet consumable part of the meter. It includes:
The Reference Electrode: This electrode is a marvel of consistency and stability. It contains a reference material in a potassium chloride solution, like mercury or mercury chloride.
The pH Glass Electrode: The sensor or indicator electrode is a glass bulb exquisitely sensitive to hydrogen ions. Millivolt output changes with hydrogen ion concentration within and outside the bulb, giving exact pH measurements.
The Amplifier
The sound system’s amplifier, the voltage amplifier in a pH meter, magnifies its accuracy. Boosting the signal ensures that the meter can accurately reflect the acidity, basicity, or neutrality level in a solution across the full pH spectrum, from 0 to 14.
Thermometer Probe
Some ph water meters come equipped with a thermometer probe, enhancing their capabilities. This feature, known as Automatic Temperature Compensation (ATC), allows the meter to account for the temperature of the solution, an important factor as temperature directly influences pH levels. This integration ensures that readings are accurate and relevant to the solution’s current state.

1. Ensure all samples reach the same temperature before measurement, as pH readings are affected by temperature. Adjust for temperature discrepancies if samples aren't at 25 °C. Use a thermometer to determine each sample's temperature and input this manually into the meter, or utilize an ATC (Automatic Temperature Compensation) probe for automatic temperature input.
2. Open the sample beakers and get them ready for testing.
3. Before placing the pH electrode in the sample, rinse it with deionized water. To avoid contaminating the samples, perform this rinsing over a waste beaker. Never use the same beaker for rinsing the electrode that you'll use for sample measurement.
4. Submerge the electrode in the first sample beaker, ensuring the tip and junction are fully immersed. Stir the sample gently and evenly.
5. Activate the meter to start recording the pH reading.
6. Wait for 1 to 2 minutes until the reading stabilizes, then record the pH and temperature of the sample.
7. For additional samples, repeat the steps from rinsing the electrode (step 3) to recording the pH and temperature (step 6). For consistent accuracy, immerse the electrode to the same depth in each sample. After completing all measurements, clean the electrode with deionized water and store it in a pH electrode storage solution.
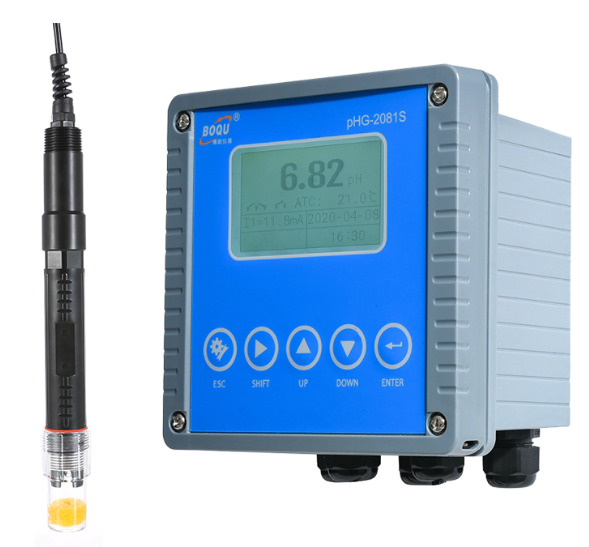
Now that you know pretty much everything about a pH meter, you must find a reliable place to buy it from. Boqu Instruments is here to make it easy for you with top-notch testing instruments and more. Visit our website to know more.
Copyright © 2019 Shanghai BOQU Instrument Co.,Ltd | All Rights Reserved
Hello, please leave your name and email here before chat online so that we won't miss your message and contact you smoothly.