Comprehensive Working Principle of Industrial pH Meter for Beginners
A pH meter is a critical instrument used to measure the acidity or basicity of a solution, which is crucial in various industrial applications. The term pH stands for potential of hydrogen, referring to the concentration of hydrogen ions (H+) in a solution. Understanding and controlling the pH levels are vital for maintaining optimal conditions in numerous industrial processes, such as water treatment, chemical manufacturing, food and beverage production, and pharmaceuticals.
Understanding pH Levels and Their Measurement
The pH scale ranges from 0 to 14, where 7 is neutral. Solutions below 7 are acidic, with lower pH values indicating higher acidity. Solutions above 7 are basic or alkaline, with higher pH values indicating stronger alkalinity. In industrial settings, precise pH control is essential for ensuring compliance with regulatory standards, enhancing product quality, and maintaining process efficiency.
Understanding the pH Scale
The pH scale is logarithmic, meaning a difference of one pH unit represents a tenfold difference in hydrogen ion concentration. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4. This logarithmic nature makes the pH scale a powerful tool for precise measurement and control.
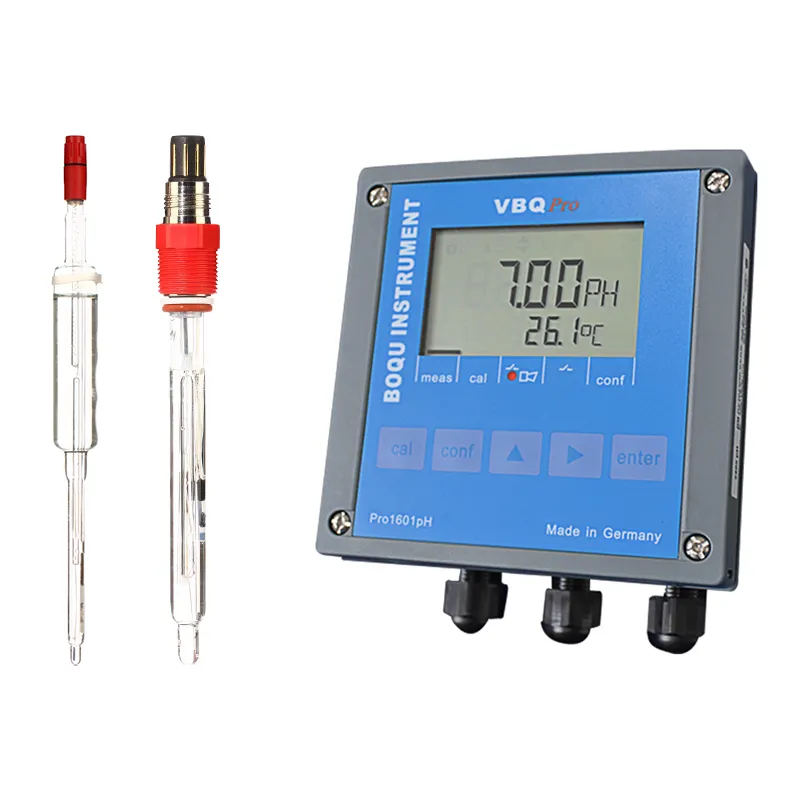
Working Principle of Industrial pH Meter
An industrial pH meter comprises several key components, each playing a crucial role in measuring and displaying accurate pH readings.
Core Components of pH Meters
- Electrode: The pH electrode is the core component, consisting of a glass bulb with a permeable membrane. This membrane allows hydrogen ions to pass through while maintaining electrical contact with the solution.
- Reference Electrode: This electrode is placed in the reference solution, providing a stable and known reference point for comparison.
- Millivolt Meter: This device measures the potential difference (in millivolts) between the pH electrode and the reference electrode, which corresponds to the pH value of the solution.
Signal Generation and Comparison
- Signal Generation: The pH electrode generates an electrical potential based on the concentration of hydrogen ions in the solution.
- Signal Comparison: The reference electrode provides a stable reference point, allowing the millivolt meter to compare the potential difference.
- Reading Display: The millivolt meter converts this potential difference into a readable pH value, typically displayed on a digital screen.
Calibration Process for Industrial pH Meters
Calibration is essential to ensure the accuracy and reliability of pH meter readings. Regular calibration helps maintain consistent and precise measurements.
Calibration Steps
- Select Standard Solutions: Use standard solutions of known pH values, such as pH 4.01, 7.00, and 10.01 buffer solutions.
- Prepare the pH Meter: Ensure the pH meter is turned on and set to the appropriate calibration mode.
- Submerge the Electrode: Immerse the pH electrode in the first standard solution while stirring gently.
- Adjust Zero Point: Adjust the meter to match the pH value of the standard solution.
- Submerge Electrode in Next Solution: Rinse the electrode with distilled water and submerge it in the next standard solution.
- Adjust Slope: Adjust the meter to match the pH value of the second standard solution.
- Final Check: Verify the meters accuracy by checking it with the third standard solution.
Applications of Industrial pH Meters in Different Industries
Industrial pH meters are widely used in various sectors, ensuring optimal conditions for production processes.
Food and Beverage Industry
In the food and beverage industry, pH meters are crucial for monitoring and controlling pH levels in fermentation and preservation processes. For instance, maintaining a consistent pH level during fermentation can enhance flavor development and improve the overall quality of the final product. Proper pH control ensures that the shelf life of acidic foods like pickles and sauerkraut is extended.
Pharmaceutical Industry
In pharmaceuticals, maintaining optimal pH conditions is essential for drug synthesis and stability testing. For example, certain active pharmaceutical ingredients (APIs) are only stable within a specific pH range. Ensuring precise pH control in manufacturing processes can significantly improve drug efficacy and patient safety.
Water Treatment
Water treatment facilities use pH meters to ensure water quality by monitoring and adjusting pH levels to comply with regulatory standards. For instance, adjusting the pH of drinking water can reduce the corrosiveness of the water, preventing lead and copper contamination from pipes.
Chemical Manufacturing
In chemical manufacturing, pH meters are used to control pH levels in chemical reactions to enhance yield and efficiency. Proper pH control can lead to higher conversion rates and better product quality. For example, in the production of cleaning agents, maintaining the right pH can improve the effectiveness of the products.
Factors Affecting the Accuracy of Industrial pH Meters
Several factors can impact the accuracy of pH meter readings.
Temperature
Temperature affects the ion activity and the response time of the electrode. For example, colder temperatures can slow down the electrodes response, while warmer temperatures can accelerate ion activity. Compensation adjustments are necessary in temperature-sensitive applications.
Contamination
Environmental contaminants can affect the electrodes performance. Regular cleaning and maintenance are essential to prevent contamination. Using distilled water for rinsing and cleaning can help maintain electrode integrity.
Electrode Aging
Electrodes degrade over time and may need replacement. Routine checks and calibrations help identify aging electrodes. Symptoms of aged electrodes include sluggish response times and inaccurate readings.
Interference
External factors like humidity and electrical fields can interfere with the readings. Shielding and environmental controls are necessary to minimize these interferences. For example, using a shielded pH electrode or a shielded cable can protect against electrical field interference.
Maintenance and Troubleshooting of Industrial pH Meters
Proper maintenance and regular troubleshooting can extend the lifespan of industrial pH meters and ensure accurate readings.
Routine Cleaning
Clean the electrode with distilled water and a soft brush to remove any contaminants. Avoid using abrasive materials that can damage the glass membrane.
Regular Calibration
Follow the calibration process at regular intervals to maintain accuracy. Calibration should be performed before each use or after any significant changes in the measurement environment.
Environmental Conditions
Monitor and control the environmental factors that can affect pH readings, such as temperature and humidity. Keeping the pH meter in a controlled environment can help maintain consistent readings.
Common Troubleshooting Tips
- No Reading: Check the connections and calibration. Ensure the electrode is properly immersed and clean. Bubbles or air pockets can interfere with the reading.
- Inconsistent Readings: Check for electrode aging or contamination. Adjust temperature compensation if necessary. Inconsistent readings can also be caused by changes in the solutions ionic strength.
- High Drift: Verify the quality of the standard solutions and calibration procedure. High drift can indicate a malfunction in the electrode or the meter. Regular calibration and replacement of aged electrodes can help mitigate this issue.
By understanding the working principle of industrial pH meters and following best practices for calibration, maintenance, and troubleshooting, you can ensure accurate and reliable pH measurements in various industrial applications. Accurate pH control is not just important but crucial for maintaining optimal conditions and ensuring product quality and safety.
-
-
-
-
-
-
5.1Temperature
-
5.4Interference
-
-
Contact Us
Office Add:No. 118 Xiuyan Road,Pudong New Area,Shanghai,Zip Code:201315,China
Contact us right away
BOQU Instrument focus on development and production of water quality analyzers and sensors, including water quality meter, dissolved oxygen meter, pH sensors, etc.
