How Calibration Affects pH Analyzer Accuracy
Understanding the Role of Calibration in pH Analyzers
Calibration for pH analyzers involves configuring the device to account for its specific sensor and operating environment. It ensures that the instrument provides accurate readings by compensating for factors like temperature, pressure, and instrument wear. Proper calibration is essential because even the most advanced pH analyzer can drift over time, leading to inaccurate results.
Simplifying Complex Terms:
- Temperature Compensation: Ensures the sensors response remains consistent across different temperatures.
- Sensor Wear: Regular calibration helps account for sensor degradation and extend its lifespan.
- Instrument Wear: Compensation for wear and tear ensures the analyzer remains reliable over time.
Calibration affects pH analyzer accuracy by ensuring that the instruments readings are true to the actual pH of the sample. This process involves setting the instrument's zero point, range, and other parameters to match the specific characteristics of the sensor. Without calibration, the instrument may provide readings that are consistently off, leading to incorrect conclusions and potential operational issues.
The Direct Impact of Calibration on pH Analyzer Accuracy
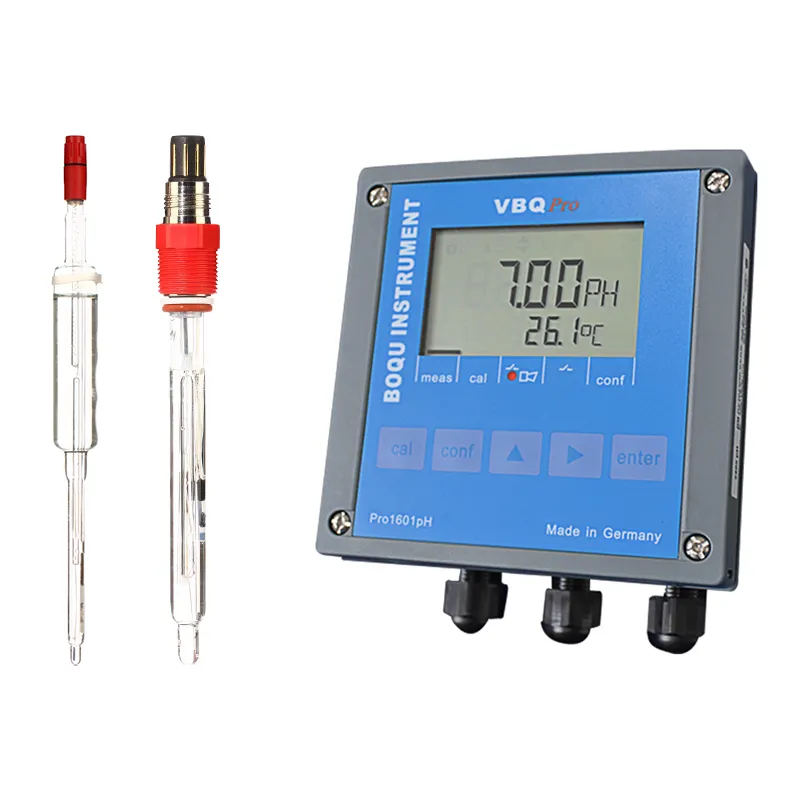
Proper calibration directly impacts the accuracy of pH measurements. The process involves several key components, including the sensor, which detects hydrogen ions, and the electronics, which convert these signals into pH readings. Calibration ensures these components function optimally.
Zeroing and Offset Calibration:
- Zeroing: Sets the pH reading to zero at the lower limit of the pH scale.
- Offset: Adjusts the reading to the upper limit to ensure a full range of accuracy.
Range Calibration:
- Ensures the instrument operates correctly over a wide range of pH values. For example, if the samples pH falls outside the instruments calibration range, the readings will be inaccurate.
Sample preparation, such as ensuring the correct volume and pH gradient, also plays a crucial role in obtaining accurate measurements. For instance, in a water treatment plant, inaccurate pH levels can result in suboptimal treatment processes, leading to untreated contaminants in the water supply. Regular calibration would restore precise measurements, ensuring that the plant operates efficiently and meets regulatory standards.
Case Study: Real-World Benefits of Regular Calibration
Before implementing a calibration schedule, a water treatment plant experienced inconsistent pH readings, leading to inefficient operations. The plants water treatment process was not consistently meeting the required pH levels, resulting in a higher incidence of untreated contaminants in the water supply. The plants pH analyzer readings varied widely, making it difficult to ensure water quality compliance.
After implementing a regular calibration schedule, the plant saw significant improvements. The pH readings became more consistent, and the treatment process became more reliable. The plant was able to meet regulatory standards and ensure better water quality, leading to cost savings and a safer product. The plants water quality compliance rate increased by 85%, and operational downtime reduced by 30%.
Best Practices for Maintaining Calibration on a pH Analyzer
Regular calibration is key to maintaining long-term accuracy. A recommended calibration schedule depends on the frequency of use and environmental conditions. For example:
- Laboratory Instrument: Calibrate every month.
- Industrial Analyzer: Calibrate weekly.
Training and awareness among personnel are also crucial. Employees should understand the importance of calibration and how to perform it correctly. Post-calibration checks, such as verifying the instrument's accuracy against known standards, help ensure ongoing reliability. Regular training sessions and the development of a calibration checklist can further enhance the calibration process.
Troubleshooting Common Issues in Calibrated pH Analyzers
Common issues include sensor drift and software errors.
- Software Errors: Can arise from outdated firmware or incorrect settings. Regular updates and quality checks can prevent these issues.
Specific Fixes: - Sensor Cleaning: Regular cleaning and proper storage conditions can help maintain sensor accuracy.
- Software Updates: Ensure the instrument's firmware is up to date and check for any software errors during calibration.
A Call to Action
Calibration is a vital process that directly impacts the accuracy of pH analyzer measurements. It ensures that the instrument provides reliable and precise readings, which are essential in various industries for maintaining operational efficiency. Neglecting calibration can lead to costly errors and operational inefficiencies, as seen in the water treatment plant case study.
In conclusion, calibration is not just a routine maintenance task; it is a critical factor that ensures the accuracy and reliability of pH measurements. By understanding the role of calibration and implementing best practices, professionals can maintain the performance of their pH analyzers and achieve accurate results. Start today to enhance the reliability of your pH measurements and ensure operational excellence.
Contact Us
Office Add:No. 118 Xiuyan Road,Pudong New Area,Shanghai,Zip Code:201315,China
Contact us right away
BOQU Instrument focus on development and production of water quality analyzers and sensors, including water quality meter, dissolved oxygen meter, pH sensors, etc.
