Manufacturer's Guide to Accurate pH Analyzer Calibration and Usage
In the world of industrial processes, precision is key. Whether you're in pharmaceuticals, water treatment, or food production, accurate pH monitoring can make all the difference. pH analyzers are indispensable tools for measuring the acidity or alkalinity of water-based solutions, ensuring that processes run smoothly and that products meet the highest standards.
pH is a critical parameter that affects numerous industrial processes and environmental systems. It measures the hydrogen ion concentration in a solution on a logarithmic scale. A pH of 7 is considered neutral; values below 7 indicate acidity, while values above 7 indicate alkalinity. In industrial settings, precise pH measurements are essential for maintaining optimal conditions. For example, in the pharmaceutical industry, pH plays a critical role in the stability of drugs and the effectiveness of formulation processes. Inadequate pH control can lead to subpar products and FDA noncompliance.
Understanding pH Measurement and its Impact
The Significance of pH in Industrial Applications
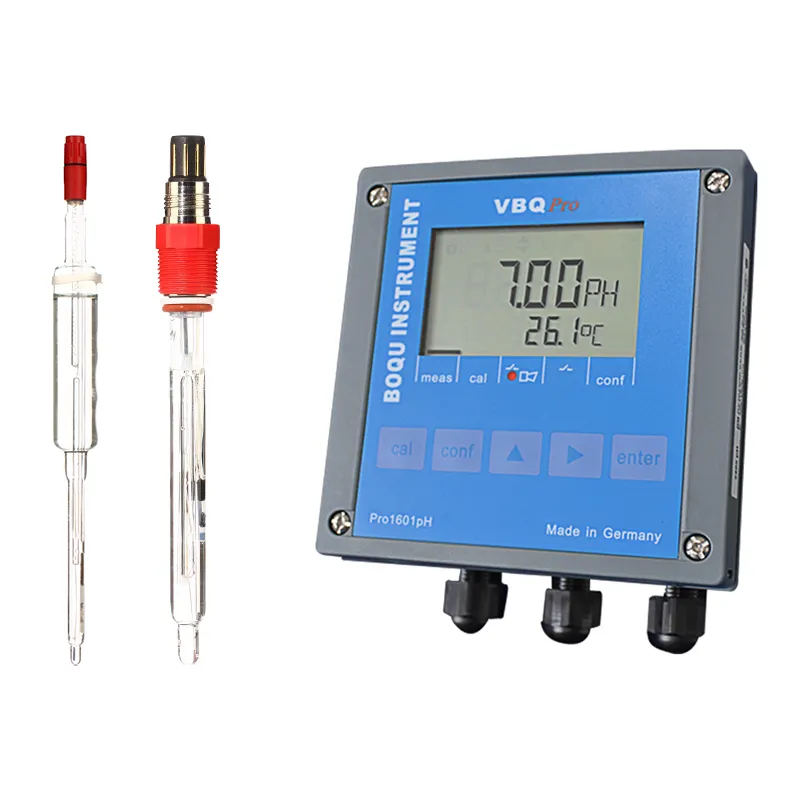
Paying close attention to pH levels is crucial for a wide range of industries. Here are a few specific examples:
- Pharmaceutical Manufacturing: Ensuring the correct pH is vital for the stability and efficacy of drugs. For instance, a slight deviation in pH can affect the rate of drug dissolution, leading to inconsistencies in dosage.
- Water Treatment: Monitoring pH is essential for ensuring water is safe and effective. Adjusting pH can prevent scaling and corrosion in pipes, making the treatment process more efficient and sustainable.
- Food Production: pH controls the growth of microorganisms, ensuring food safety. For example, a pH of 4.6 or lower is required for most foods to inhibit the growth of pathogens like E. coli and Listeria.
Proper Calibration Steps for pH Analyzers
Calibration is the cornerstone of accurate pH analyzer performance. Ensuring that your pH analyzer is properly calibrated can significantly enhance the reliability of your process control and monitoring. Here are the essential steps to follow:
1. Select Reference Electrodes: Choose a reference electrode that is compatible with your pH sensor and meets the required specifications for your application.
2. Prepare Calibration Solutions: Use standard buffer solutions that are accurate and stable. Commonly used buffers include pH 4.01, 7.00, and 10.01, which represent acidic, neutral, and basic conditions, respectively.
3. Calibrate the pH Sensor: Submerge the pH sensor in the calibration solution, allowing it to stabilize. Adjust the transmitter to match the known pH value of the solution.
4. Repeat with Additional Buffers: To ensure accuracy over a wide range, repeat the calibration process with at least two different buffer solutions.
5. Use a High-Quality Transmitter: Ensure that the transmitter is compatible with the pH sensor and can provide accurate real-time readings.
Factors Affecting pH Measurement Accuracy
Common Factors Influencing pH Readings
While calibration is essential, maintaining accuracy also depends on several other factors that can affect pH measurements. Here are some key factors to consider:
- Temperature Fluctuations: Temperature variations can significantly affect pH readings. Ensure that the pH sensor and buffer solutions are at the same temperature.
- Electrode Condition: Over time, pH electrodes can become contaminated or degrade. Regular cleaning and maintenance are crucial to keeping the sensor in optimal condition.
- Environmental Conditions: Humidity, pressure, and other environmental factors can also influence pH readings. Controlling these conditions can minimize measurement errors.
Maintaining Consistent Environmental Conditions
To ensure consistent and accurate pH measurements, its essential to maintain consistent environmental conditions. Here are some practical tips:
- Controlling Temperature: Use a temperature monitor and adjust the temperature if necessary to keep it stable.
- Protecting the Sensor: Keep the sensor shielded from physical damage and ensure it is not exposed to harsh chemicals or excessive pressure.
- Optimizing Flow Rates: Ensure proper flow rates in the process to avoid any variations in the pH readings.
Best Practices for pH Analyzer Usage
Practical Tips for Using pH Analyzers
Proper usage of pH analyzers involves following best practices specific to the industrial environment in which they are deployed. Here are some key tips:
- Regular Maintenance: Perform routine maintenance, such as cleaning and calibration, to ensure optimal performance.
- Proper Installation: Follow manufacturer guidelines for installation to ensure that the pH sensor is correctly positioned and protected.
Reducing Errors and Maintaining Consistent Data
- Avoid Cross-Contamination: Keep calibration solutions and samples separate to prevent contamination.
- Regular Calibration: Calibrate your pH analyzer regularly, even if it seems to be functioning correctly.
- Use the Right Reagents: Ensure that you are using the correct reagents and buffer solutions for your specific application.
Troubleshooting Common Issues with pH Analyzers
Common Problems and Solutions
Even with proper calibration and usage, pH analyzers can still face various issues. Here are some common problems and their solutions:
- Incorrect Calibration: Double-check your calibration process, ensuring that you are using the correct solutions and following the manufacturers guidelines.
- Contaminated Electrodes: Clean the electrodes regularly, and if contamination persists, you may need to replace the sensor.
- Inaccurate Readings: Check environmental factors such as temperature and pressure, and ensure that the sensor is installed correctly.
Effective Troubleshooting Techniques
To resolve common issues effectively, follow these steps:
1. Identify the Issue: Determine the specific problem based on the readings and symptoms.
2. Check Calibration: Ensure that the pH sensor and transmitter are properly calibrated.
3. Inspect the Sensor: Clean or replace the sensor if contamination or damage is suspected.
4. Monitor Environmental Factors: Adjust environmental conditions to maintain optimal performance.
Comparison of pH Measurement Methods
Traditional vs. Modern Techniques
Modern pH analyzers offer significant advantages over traditional methods. Heres a comparative analysis:
- Traditional Methods:
- Ion Selective Electrodes (ISE): These are reliable but require manual calibration and can be affected by temperature and other factors.
- Electronic pH Meters: These are highly portable and convenient but may not be as accurate as modern analyzers.
- Modern Techniques:
- Optical pH Sensors: These offer higher precision and sensitivity, making them ideal for critical applications.
- Integrated pH Analyzers: These provide automated calibration and data logging, ensuring continuous and accurate monitoring.
Advantages and Disadvantages
- Optical pH Sensors:
- Advantages: High precision, easier to use, automatic calibration, and extended stability.
- Disadvantages: Higher initial cost, requires regular calibration to maintain accuracy.
- Integrated pH Analyzers:
- Advantages: Automated calibration, real-time data logging, improved accuracy, and ease of use.
- Disadvantages: Higher cost, more complex setup and maintenance.
Successful Implementation of pH Analyzers
Real-World Examples
Successful implementation of pH analyzers can lead to significant improvements in process efficiency and product quality. Here are some real-world examples:
- Pharmaceutical Manufacturing: A large pharmaceutical company integrated an advanced pH analyzer into their process to ensure consistent product quality and regulatory compliance.
- Water Treatment: A water treatment plant used a pH analyzer to monitor the pH of their process water, leading to a more efficient and sustainable treatment process.
Insights from Industrial Success Stories
These case studies highlight the importance of accurate pH measurements and the benefits of using advanced pH analyzers. By adopting best practices and maintaining regular calibration, industries can achieve greater precision and reliability in their processes.
Future Trends in pH Analyzers
Emerging Technologies and Advancements
The future of pH analyzers looks promising with emerging technologies and advancements. Here are some potential developments:
- IoT Integration: pH analyzers can be integrated with Internet of Things (IoT) systems for real-time monitoring and remote access.
- Artificial Intelligence: AI can be used to predict and prevent issues, optimize calibration, and enhance overall performance.
- Miniaturization: Smaller, more portable pH analyzers are being developed for on-site and field applications.
Conclusion
In conclusion, accurate and reliable pH measurements are essential for maintaining quality, efficiency, and safety in industrial processes. By understanding the fundamentals of pH measurement, implementing best practices, and staying informed about emerging technologies, manufacturers can ensure that their pH analyzers deliver the precision needed for success.
Embrace the advancements in pH analyzer technology and stay ahead of the curve to achieve consistent and accurate pH measurements. Whether youre in pharmaceuticals, water treatment, or any other industry, precise pH control is key to meeting your quality and regulatory standards.
Contact Us
Office Add:No. 118 Xiuyan Road,Pudong New Area,Shanghai,Zip Code:201315,China
Contact us right away
BOQU Instrument focus on development and production of water quality analyzers and sensors, including water quality meter, dissolved oxygen meter, pH sensors, etc.
